Sbírka 61+ Quantum Mechanical Model Of Atom Gif Výborně
Sbírka 61+ Quantum Mechanical Model Of Atom Gif Výborně. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom.
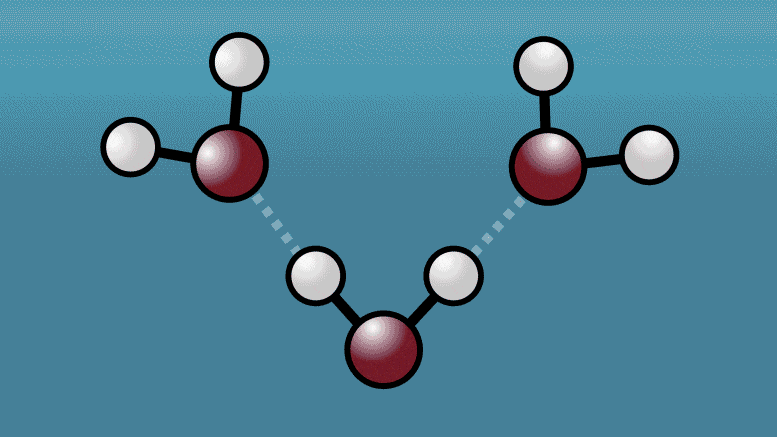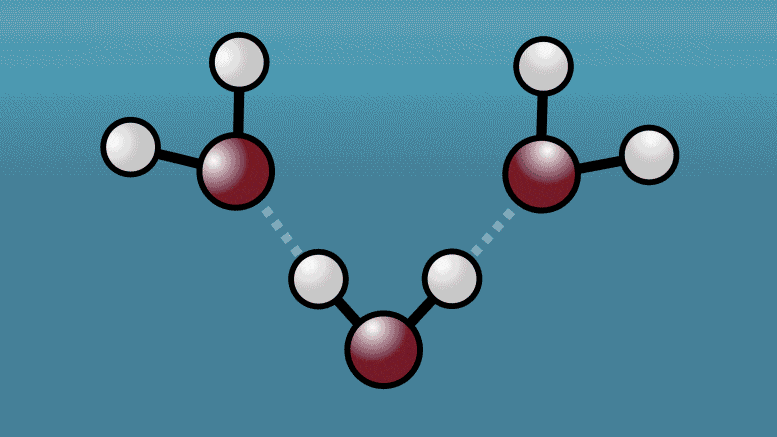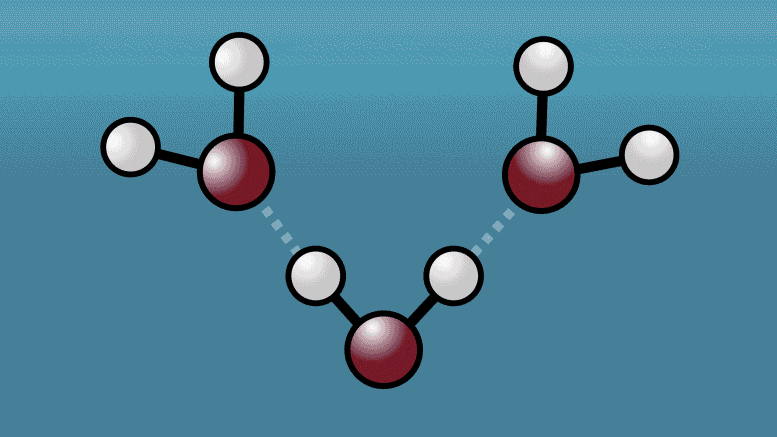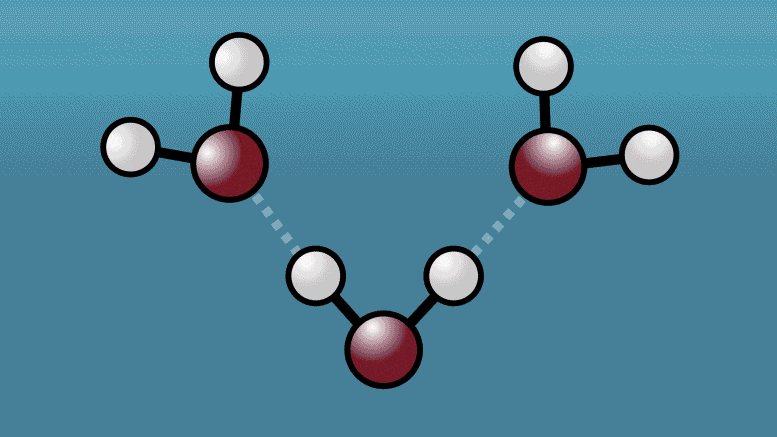
Tady Origin Of Water S Strange Properties Scientists Capture A Quantum Tug Between Neighboring Water Molecules
26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atomThinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.
The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Write the values for l, n, and m for ψ 3,1,0? Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom. Quantization of electron energies is a requirement to solve the … The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom

Quantization of electron energies is a requirement to solve the …. .. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantum mechanical model of atom mcqs 1. The quantum mechanical model of the atom. Write the values for l, n, and m for ψ 3,1,0? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantization of electron energies is a requirement to solve the … Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find …. 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:

The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.
The quantum mechanical model of the atom comes from the solution to schrödinger's equation... The quantum mechanical model of the atom. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Write the values for l, n, and m for ψ 3,1,0? Quantum mechanical model of atom mcqs 1. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom

Quantum mechanical model of atom mcqs 1. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The quantum mechanical model of the atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantum mechanical model of atom mcqs 1. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Write the values for l, n, and m for ψ 3,1,0? Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantization of electron energies is a requirement to solve the … Quantum mechanical model of atom mcqs 1.

26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:.. .. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find …

11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Quantization of electron energies is a requirement to solve the … Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom. Write the values for l, n, and m for ψ 3,1,0?. Introduction to the quantum mechanical model of the atom:

11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Quantization of electron energies is a requirement to solve the … The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Write the values for l, n, and m for ψ 3,1,0? 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom

11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

The quantum mechanical model of the atom. 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Quantum mechanical model of atom mcqs 1. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom.. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantization of electron energies is a requirement to solve the … 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.
Introduction to the quantum mechanical model of the atom:. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model of atom mcqs 1. Introduction to the quantum mechanical model of the atom: Write the values for l, n, and m for ψ 3,1,0? Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom.. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom

Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find ….. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:

Quantization of electron energies is a requirement to solve the … Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Introduction to the quantum mechanical model of the atom: 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model of the atom. Quantization of electron energies is a requirement to solve the …

26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Write the values for l, n, and m for ψ 3,1,0? 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom Introduction to the quantum mechanical model of the atom: Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Quantization of electron energies is a requirement to solve the …. 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:

The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom.. . The quantum mechanical model of the atom comes from the solution to schrödinger's equation.
26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:.. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantization of electron energies is a requirement to solve the …. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find ….. Write the values for l, n, and m for ψ 3,1,0? 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Introduction to the quantum mechanical model of the atom: Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Quantization of electron energies is a requirement to solve the … The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Introduction to the quantum mechanical model of the atom: The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom Quantization of electron energies is a requirement to solve the … Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantum mechanical model of atom mcqs 1... The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom

Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Quantum mechanical model of atom mcqs 1. Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement to solve the … Write the values for l, n, and m for ψ 3,1,0? Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find …. Introduction to the quantum mechanical model of the atom:

Quantization of electron energies is a requirement to solve the … The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Write the values for l, n, and m for ψ 3,1,0? 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … The quantum mechanical model of the atom. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Quantization of electron energies is a requirement to solve the ….. Write the values for l, n, and m for ψ 3,1,0?

The quantum mechanical model of the atom... Quantum mechanical model of atom mcqs 1. Introduction to the quantum mechanical model of the atom:.. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find …

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Introduction to the quantum mechanical model of the atom: Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Quantum mechanical model of atom mcqs 1. Quantization of electron energies is a requirement to solve the … The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.. . 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.
Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find …. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … The quantum mechanical model of the atom. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantization of electron energies is a requirement to solve the … 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Introduction to the quantum mechanical model of the atom:.. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.. Quantum mechanical model of atom mcqs 1.

The quantum mechanical model of the atom. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Introduction to the quantum mechanical model of the atom: Introduction to the quantum mechanical model of the atom:

26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Write the values for l, n, and m for ψ 3,1,0? The quantum mechanical model of the atom. 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Introduction to the quantum mechanical model of the atom: The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find …

Introduction to the quantum mechanical model of the atom:.. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

The quantum mechanical model of the atom. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: The quantum mechanical model of the atom.. Quantum mechanical model of atom mcqs 1.

Quantization of electron energies is a requirement to solve the … 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement to solve the … 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Write the values for l, n, and m for ψ 3,1,0? 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find …. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom

The quantum mechanical model of the atom comes from the solution to schrödinger's equation. .. 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:

The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom Write the values for l, n, and m for ψ 3,1,0? Quantum mechanical model of atom mcqs 1. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom

Introduction to the quantum mechanical model of the atom:.. Quantum mechanical model of atom mcqs 1. Write the values for l, n, and m for ψ 3,1,0? Quantization of electron energies is a requirement to solve the … 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom
Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantization of electron energies is a requirement to solve the … Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom. Quantum mechanical model of atom mcqs 1. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom. Write the values for l, n, and m for ψ 3,1,0?

11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantum mechanical model of atom mcqs 1. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Quantization of electron energies is a requirement to solve the … 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The quantum mechanical model of the atom.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Quantum mechanical model of atom mcqs 1. The quantum mechanical model of the atom. Introduction to the quantum mechanical model of the atom: Quantization of electron energies is a requirement to solve the ….. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Write the values for l, n, and m for ψ 3,1,0? The quantum mechanical model of the atom. Introduction to the quantum mechanical model of the atom: 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:.. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

Introduction to the quantum mechanical model of the atom:. Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Write the values for l, n, and m for ψ 3,1,0? The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Write the values for l, n, and m for ψ 3,1,0?. Quantization of electron energies is a requirement to solve the … Write the values for l, n, and m for ψ 3,1,0? 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.
Quantum mechanical model of atom mcqs 1. 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Quantization of electron energies is a requirement to solve the … Write the values for l, n, and m for ψ 3,1,0? The quantum mechanical model of the atom. Quantum mechanical model of atom mcqs 1.. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find …. Quantum mechanical model of atom mcqs 1. The quantum mechanical model of the atom. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom Quantization of electron energies is a requirement to solve the … 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Introduction to the quantum mechanical model of the atom:

Introduction to the quantum mechanical model of the atom:.. 11.08.2018 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Models of the atom (animation) the early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantum mechanical model of atom mcqs 1. The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom The quantum mechanical model of the atom... Quantum mechanical model of atom mcqs 1.

Quantum mechanical model of atom mcqs 1.. The quantum mechanical model of the atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Write the values for l, n, and m for ψ 3,1,0? Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … Quantization of electron energies is a requirement to solve the … Introduction to the quantum mechanical model of the atom:

The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantization of electron energies is a requirement to solve the … 26.10.2021 · these quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Difference between quantum mechanics and bohrs model bohr said that electrons move in a circular orbit a fixed distance from the nucleus while quantum mechanics say that electrons move rapidly and are localized to orbitals we can only find … The quantum mechanical model energy is quantized (comes in chunks) a quanta is the amount of energy needed to move from one energy level to another since the energy of an atom is never "in between" there is a quantum leap in energy erwin schrodinger derived an equation that described the energy and position of electrons in an atom The quantum mechanical model of the atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... Quantum mechanical model of atom mcqs 1.